September 21, 2020 • 5 min read
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel betacoronavirus that causes coronavirus disease 2019 (COVID-19). SARS-CoV-2 has a genome sequence highly similar to SARS-CoV and was initially transmitted across species with subsequent human-to-human transmission like SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).1 SARS-CoV-2 has caused a global pandemic with nearly 3.5 million cases and more than 240,000 deaths worldwide as of May 3, 2020.2 As of early May, the United States has more than 1.1 million confirmed cases, with more than 25 percent of these identified in New York.3 Differential incidence in select regions has also been observed in other parts of the world with important implications for available healthcare resources and outcomes in these geographic areas.
The transmissibility of SARS-CoV-2 has been an area of intense focus. Initial estimates of the basic reproduction number (Ro) ranged from 1.4 to 5.5 compared with the 2009 H1N1 and 1918 H1N1 Ro ranges of 1.2 to 1.6 and 1.4 to 3.8, respectively.4 COVID-19 has propagated through some communities and geographic areas easily and rapidly. Scientific understanding of transmissibility, asymptomatic and pre-symptomatic transmission, and measures that effectively reduce transmission continues to evolve. Nonpharmaceutical interventions (NPIs) include hand hygiene, isolation of cases and asymptomatic exposures, social distancing, closures of schools, limits on gatherings (e.g., sporting events, festivals, etc.), and the cleaning of shared surfaces (Figure 1).5 NPIs can limit the spread of infection and are especially important for novel pandemic viruses because effective vaccines, efficacious treatments, and population immunity do not exist in the initial phase of the pandemic. The estimated effect of NPIs can depend on the timing, type(s), number, and duration of implementation.6 Importantly, the strategy for implementing community NPIs may influence the overall number of cases and affect the likelihood of exceeding available healthcare resources.7
Figure 1 | Non-pharmaceutical interventions for COVID-19

As more countries have large numbers of individuals infected, our understanding of the clinical course, complications, and resolution of COVID-19 continues to evolve. Clinical presentations are highly variable and include mild upper respiratory infection, gastrointestinal symptoms, loss of taste or smell, or acute respiratory distress syndrome. The current understanding is that the mortality is higher than influenza, but lower than SARS-CoV or MERS. Additional time is needed to fully understand mortality associated with COVID-19 as there has been insufficient time to observe outcomes in a large number of individuals, fully evaluate ascertainment bias (i.e., outcomes ascertained primarily in those with the most severe disease), and understand differences in attribution of deaths to COVID-19 across countries.8 Nevertheless, mortality is higher asage increases and with preexisting chronic disease (e.g., diabetes, cardiovascular disease) or when healthcare systems are overwhelmed.
Scientific efforts have included identification of efficacious therapies and vaccine development. A phase 1 COVID-19 vaccine clinical trial started in the United States in March 2020, and numerous vaccine efforts are in progress throughout the world. A number of potential treatments for COVID-19 are currently under evaluation. Potential therapies for COVID-19 involve a spectrum of therapeutics with different mechanisms of action and include remdesivir, ydroxychloroquine/chloroquine, and antibodies from convalescent plasma. Rigorous study and evidence are needed to understand the efficacy and safety of potential treatments.9 The vast majority of initial clinical trials exclude individuals with advanced kidney disease, greatly limiting the understanding of optimal treatment strategies for this highly vulnerable population. More trials that include this population are urgently needed as these individuals may be at highest risk for disease complications and for serious adverse events related to potential therapies.
Many countries have been faced with extraordinary challenges as they experienced an exponential increase in COVID-19 cases. The number of people with COVID-19 in need of intensive medical care exceeded or strained available healthcare resources in some geographic areas. As the pandemic has evolved, societal functions throughout the world have changed dramatically. The changes to social structure and function on a scale of this magnitude have been difficult, but these extraordinary measures have been undertaken to slow the spread of the pandemic.
There is great uncertainty as the pandemic evolves. In those countries that have already successfully suppressed SARS-CoV-2, there is potential for a reemergence.10 In those communities that have not suppressed SARS-CoV-2, but have observed a reduction or plateauing of cases, there is significant debate about how to modify community NPIs without initiating a second wave of infections. The coming year will be critical as the risk for a resurgence in infections remains—and will likely persist until the population has reached herd immunity either through infection or vaccination.
As the number of COVID-19 cases starts to decline in many communities, healthcare organizations will be faced with numerous challenges as they attempt to return to providing elective and non-urgent care for the general population while simultaneously caring for individuals with COVID-19 and remaining prepared for a potential resurgence of the virus. As countries decrease community NPIs, public health departments and healthcare organizations will have to rapidly identify and isolate new cases, trace contacts, protect the most vulnerable populations, and consider sustainable models of healthcare for the coming years. Public health officials and healthcare organizations throughout the world will need to continue to come together to learn, evolve, and effect the course of this pandemic.
PROGRESSION OF THE PANDEMIC MAY—JULY
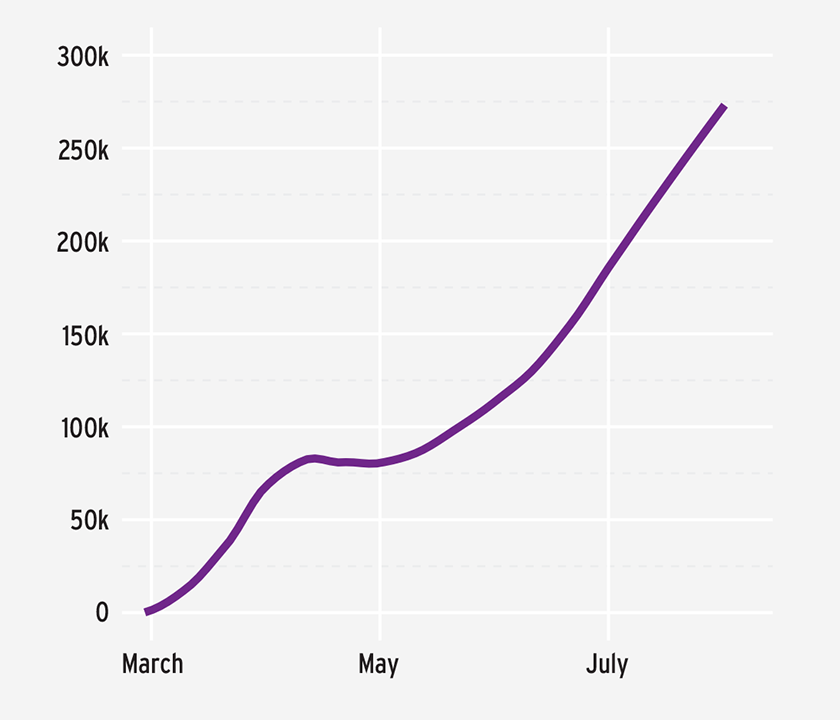
Since early May, when this chapter was originally written, the pandemic has continued to evolve, along with the understanding of the clinical course of COVID-19, the impact of NPIs, efficacious therapies, and vaccine candidates. As of late July 2020, more than 16.7 million cases of COVID-19 have been identified and over 660,000 deaths have been attributed to COVID-19 worldwide. In the United States, more than 4.3 million cases have been confirmed and nearly 150,000 deaths have been attributed to COVID-19.11 Globally, the number of cases in July far exceeded those in April, with a number of countries continuing to experience significant transmission of the virus (Figure 2).12
FIGURE 2 | Increasing new daily global COVID-19 cases
NPIs, such as contact tracing and social distancing, continue to be critically important strategies for containing or mitigating disease transmission. Emerging evidence supports the importance of universal masking to decrease the transmission of the virus (Figure 3).13,14,15 Testing remains central to slowing disease transmission and continues to vary by country with substantive differences in the scale of testing and positivity rate.16
FIGURE 3 | Universal masking

Since May, a number of clinical studies of the safety and efficacy of COVID-19 treatments have been published and have informed clinical guidelines and management of patients with COVID-19.17 Dexamethasone is now recommended for individuals with severe COVID-19 disease who are hospitalized; Remdesevir is recommended for individuals requiring supplemental oxygen or hospitalized with severe disease.18 Hydroxychloroquine has not been shown to be an effective treatment for COVID-19, and the FDA has revoked the emergency use authorization for this medicine.19,20
Globally, vaccine trials have continued to progress. For example, the findings from the phase 1 trial of the mRNA-1273 vaccine and phase 1/2 trial of the ChAdOx1 nCoV-19 vaccine were published in July.21,22 The phase 3 trial of the mRNA-1273 vaccine and late-stage phase 2/3 trials of the ChAdOx1 nCoV-19 vaccine have started.23,24
As the COVID-19 pandemic continues to evolve, so does the science and our understanding of disease transmission, longterm complications, therapeutic areas, and the adaptive immune response to COVID-19. The recent months have highlighted some of the significant challenges, including the influence of misinformation and disinformation, inadequate testing capacity, non-acceptance of NPIs (e.g., social distancing, face masks), and the balance between rapid and rigorous research. Much uncertainty remains, but as the science and our understanding of the challenges advance so must the global response to the pandemic.
Meet The Experts
References
- Chen J. Pathogenicity and transmissibility of 2019-nCoVd—a quick overview and comparison with other emerging viruses. Microbes and Infection 2020;22(2):69-71. https://doi.org/10.1016/j.micinf.2020.01.004.
- Center for Systems Science and Engineering (CSSE) at Johns Hopkins University: COVID-19 Dashboard. https://www.arcgis.com/apps/opsdashboard/index.html#/ bda7594740fd40299423467b48e9ecf6. Accessed May 3, 2020.
- Ibid.
- Chen. Pathogenicity and transmissibility of 2019-nCoVd.
- Centers for Disease Control and Prevention. Nonpharmaceutical interventions (NPIs). https://www.cdc.gov/nonpharmaceutical-interventions/index.html. Accessed March 29, 2020.
- Ferguson NM, Laydon D, Nedjati-Gilani G, et al. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. Imperial College COVID-19 Response Team report, March 16, 2020. https://sciencebusiness.net/sites/default/files/inline-files/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf.
- Ibid.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. Published online March 23, 2020. https://jamanetwork.com/journals/jama/fullarticle/2763667.
- Kalil AC. Treating COVID-19—off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. Published online March 24,2020. https://jamanetwork.com/journals/jama/fullarticle/2763802.
- Ferguson et al. Impact of non-pharmaceutical interventions.
- CSSE COVID-19 Dashboard. https://www.arcgis.com/apps/opsdashboard/index. html#/bda7594740fd40299423467b48e9ecf6. Accessed July 29, 2020.
- Ibid.
- Hendrix MJ, Walde C, Findley K, Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy—Springfield, Missouri, May 2020. MMWR 2020 July 17;69(28):930-2. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6928e2-H.pdf.
- Wang X, Ferro EG, Zhou G, et al. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA, published online July 14, 2020. https://jamanetwork.com/journals/jama/fullarticle/2768533.
- Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet 2020;395(10242):1973-87. https://www.sciencedirect.com/science/article/pii/S0140673620311429?via%3Dihub.
- Statistics and Research: Coronavirus pandemic (COVID-19). Our World in Data. Updated daily. https://ourworldindata.org/coronavirus.
- Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. IDSA, April 11, 2020 (with updates). https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-andmanagement/#toc-3. Accessed July 29, 2020.
- Ibid.
- Ibid.
- US Food & Drug Administration. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. Press release, June 15, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorizationchloroquine-and.
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARSCoV-2—preliminary report. NEJM, published online July 14, 2020. https://www.nejm.org/doi/full/10.1056/NEJMoa2022483.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, singleblind, randomised controlled trial. Lancet, published online July 20, 2020. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31604-4/fulltext.
- National Institutes of Health. Phase 3 clinical trial of investigational vaccine for COVID-19 begins. Press release, July 27, 2020. https://www.nih.gov/news-events/news-releases/phase-3-clinical-trial-investigational-vaccine-covid-19-begins.
- AstraZeneca. COVID-19 vaccine AZD1222 showed robust immune responses in all participants in phase I/II trial. Press release, July 20, 2020. https://www.astrazeneca.com/media-centre/press-releases/2020/covid-19-vaccine-azd1222-showed-robust-immune-responses-in-all-participants-in-phase-i-ii-trial.html.

