RESOURCES
References:
- Blake PG & Daugirdas JT, Physiology of Peritoneal Dialysis, Handbook of Dialysis 5th edition, pp. 392 -407.
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020.
- Nongnuch, A., Assanatham, M., Panorchan, K., Davenport, A., Strategies for preserving residual renal function in peritoneal dialysis patients, Clinical Kidney Journal, Volume 8, Issue 2, April 2015, Pages 202–211, https://doi.org/10.1093/ckj/sfu140.
- Maharjan SRS, Davenport A. Comparison of sodium removal in peritoneal dialysis patients treated by continuous ambulatory and automated peritoneal dialysis. J Nephrol. 2019 Dec;32(6):1011-1019.
- Courivard & Davenport (2015) Phosphate removal in PD; Peritoneal Dialysis International, Vol. 36, pp.85–93.
- Roumeliotis et al (2020), APD or CAPD: one glove does not fit all. Int. Urology and Nephrology, https://doi.org/10.1007/s11255-020-02678-6.
- Lambert, MC, Lage, C, Kirchgessner, J., stay•safe®. A new PVC free system. EDTNA|ERCA JOURNAL 1999 XXV 3.
- Port, F.K., P.J. Held, K.D. Knolph, M.N. Turenne, R.A. Wolfe. “Risk of Peritonitis and Technique Failure by CAPD Connection Technique: A National Study.” Kidney International 42 (1992): 967-974.
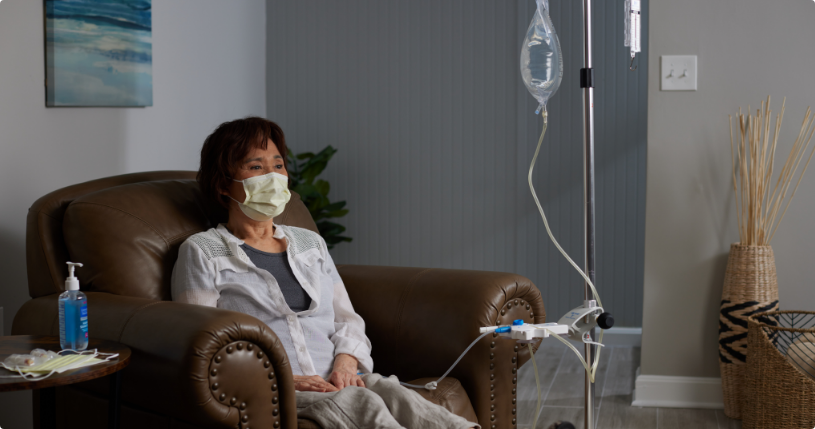
Indications for Use: DELFLEX is indicated in the treatment of chronic kidney failure in patients being maintained on peritoneal dialysis.
DELFLEX is available by prescription only.
IMPORTANT SAFETY INFORMATION
- Intended for intraperitoneal administration only
- Not for intravenous or intra-arterial administration
- Use aseptic technique throughout the procedure
- Monitor routinely for electrolyte, fluid, and nutrition imbalances
- Monitor for signs of peritonitis or overfill
- Inspect the drained fluid for fibrin or cloudiness
- Ensure that there is no leakage around the catheter
- Solution-related adverse reactions may include peritonitis, catheter site infection, electrolyte and fluid imbalances, hypovolemia, hypervolemia, hypertension, disequilibrium syndrome, muscle cramping, abdominal pain, abdominal distension, and abdominal discomfort
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Medical Care North America at 800-323-5188. You are encouraged to report negative side effects of prescription drugs to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Visit MedWatch or call 1-800-FDA-1088.
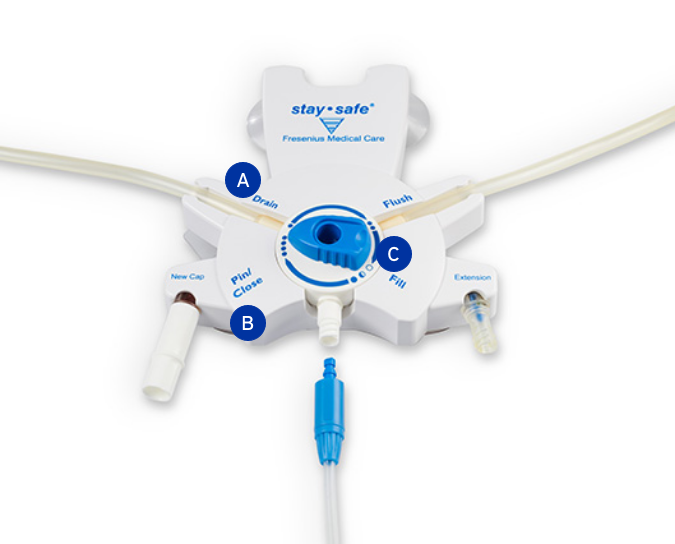
Fresenius CAPD stay•safe® Disposable Administration Sets with stay•safe® Connector:
The Fresenius CAPD stay•safe® Disposable Administration Sets with stay•safe® Connector is indicated for use in end-stage acute and chronic renal disease.
Liberty® Select
stay•safe Catheter extension set:
The stay•safe catheter extension set with Luer-Lock is indicated for use in patients with acute and chronic end-stage renal disease undergoing peritoneal dialysis (PD) in a healthcare facility or at home. The stay•safe catheter extension set with Luer-Lock is used to connect a PD catheter with a Luer-Lock catheter adapter to PD systems that use stay•safe PIN technology.
stay•safe Cap:
The Fresenius sterile stay•safe® Cap is intended to be used for closure of the stay safe stay•safe peritoneal dialysis connectology system.
Liberty Select Cycler:
Indications for Use: The Fresenius Liberty Select Cycler is indicated for acute and chronic peritoneal dialysis.
Caution: Federal (US) law restricts this device to sale by or on the order of a physician.
Note: Read the Instructions for Use for safe and proper use of this device. For a complete description of hazards, contraindications, side effects, and precautions, see full package labeling at www.fmcna.com.
©2009, 2018-2023 Fresenius Medical Care. All Rights Reserved. Fresenius Medical Care, the triangle logo, Liberty, stay·safe, and DELFLEX are trademarks of Fresenius Medical Care Holdings Inc. or its affiliated companies. All other trademarks are the property of their respective owners. P/N 100969-01 Rev F 06/2023