
Advancing Medicine by Offering Clinical Research to Patients | FMCNA
The concept of clinical trials has a long history, dating back hundreds of years; however, the process remained unstructured until 1747 when the first well-formulated clinical research project was developed to cure sailors from scurvy. James Lind, a Scottish physician, separated sick sailors into two groups. The groups had identical diets, but one group was given lemon juice. He discovered that citrus fruits cured scurvy. Since then, clinical trials have been the key pathway of medical innovation.1
In the 21st century, the utilization of clinical trials is highly variable and depends on the condition being treated. Although the investments in research and development for renal research have increased in recent years, the efforts fall far behind other medical disciplines (Figure 1).2 Oncology, for example, has actively established clinical research as a routine aspect of care, especially in breast cancer treatment and non-small cell lung cancer treatment.3 In addition, the investigators in cancer clinical trials devote efforts to educate both the patients and clinicians, so the clinical trials are well regarded by both parties.
FIGURE 1 | Top 200 Hot Indications Based on 2015 Investment Intensity: Pipeline Score, R&D Funding, and Academic Focus
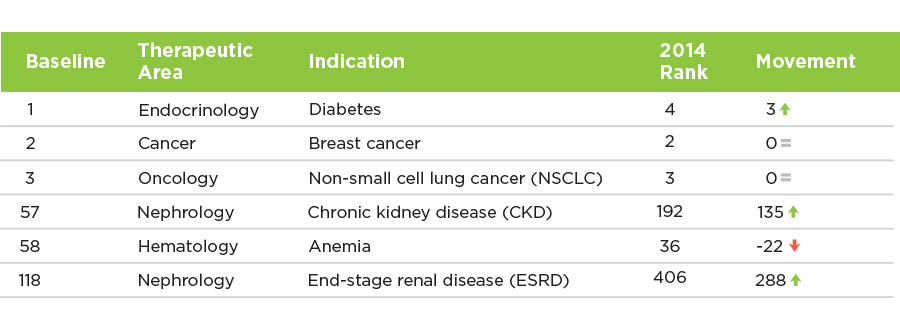
According to recent research, patients with previous clinical research participation are willing to seek out new clinical trial opportunities. Patients are also more likely to participate in clinical research when invited to do so by their physicians. This engaging environment of caregivers, researchers, and patients has driven significant advances in cancer therapies.4
Such an environment is not typical in clinical research renal therapies. This is true even though patients undergoing renal treatment have higher mortality rates than breast and prostate cancer patients. The goal at Frenova Renal Research is to advance the science of renal therapies and help bring novel treatments to market for patients with renal impairment. This is done in a manner that facilitates the introduction of approved new therapies into Fresenius Kidney Care, thereby encouraging greater participation in clinical research.
Delivering better care and improving the lives of dialysis patients are best achieved by collaborative research between academia, industry, patients, and health care providers. Building these relationships widens the availability of resources, reinforces system thinking, and improves the product by utilizing complementary skills and input.5,6 Frenova advances clinical research through collaborations with caregivers, academic researchers, pharmaceutical companies, and medical device firms. These collaborations enable the clinical research activities that are necessary to bring about innovations in patient care and novel therapies designed to improve the lives of people living with renal impairment.
Nonetheless, psychosocial barriers can often hinder medical advancements. The general population is uninformed of clinical trials and why such trials are important. In fact, only 33 percent of U.S. adults have heard of clinical trials, and, of those, fewer than 5 percent know where to find information about them.7 This lack of understanding perpetuates a generally negative perception of clinical trials. Further, when provided a fundamental education on clinical trials, patients and their families often reject them out of concerns regarding potential side effects/safety, uncertainty about insurance and out-of-pocket costs, inconvenience of trial locations, concerns about receiving a placebo, skepticism about unproven treatments, and worries over feeling like "guinea pigs."8 A concerted and comprehensive effort to educate patients and their families is key to ameliorating these issues.
One approach to improve patient perceptions of clinical trials is utilizing physician-patient interactions. A national public opinion poll performed in 2013 indicated that 80 percent of the public consider their physician recommendation very important to participating in a clinical trial. Yet, only 23 percent say a health professional has talked to them about it (Figure 2).9
FIGURE 2 | Patient preferred and actual sources for information about clinical trials
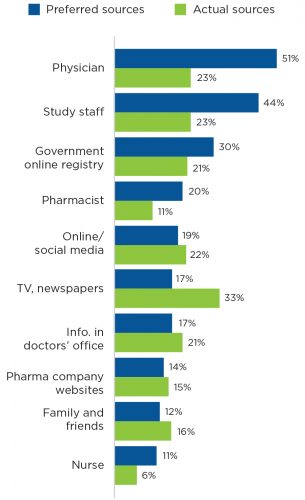
Lack of communication is a major hindrance for clinical research participation and is a barrier that the renal care community needs to overcome with collaboration, active support, patient engagement and education. Working as a team is essential to:
- Building organizational awareness and understanding about how clinical research is conducted and the important role patients play in advancing medical science through research
- Routinely initiating conversations with patients about clinical research
- Making general informational materials on research readily available
- Referring patients interested in research to investigator physicians
- Understanding that only through research can we hope to offer better care and improve the lives of our patients
The fact is that clinical research provides a symbiotic relationship for everyone. Besides the inherent benefit of developing improved therapies, clinical research has been shown to improve participants' health outcomes because they receive extra attention during a clinical trial. A consequence of extra attention is that patients tend to become more actively engaged in their own care.
A study of 494 hospitals showed that institutions that participated in clinical trials had fewer deaths from heart attacks, suggesting that patients treated at those hospitals received better care andhad better health outcomes because of their study participation.12 Correspondingly, Frenova's preliminary results suggest that Fresenius Kidney Care dialysis facilities supporting higher volumes of research activity achieved higher performance ratings on patient outcomes, patient satisfaction, and financial performance. Clinical research that results in better outcomes for renal disease patients will always translate to increased benefits for their caretakers. "We often hear from patients that they relish the dedicated time with the physicians and enjoy visiting with the coordinators when they take part in a study. This is a win-win for both sides and often leads to repeat trial participants," says Claire Meunier, Michael J. Fox Foundation's vice president of research engagement.10
Frenova continues working to bring innovation to the medical field of kidney disease care. As a component of FMCNA, which cares for a large renal patient population, Frenova is in a truly unique position to do so. As a first step, it is imperative that we acknowledge the importance of clinical research in delivering better care and learn how research benefits Fresenius Kidney Care's patients.
CONCLUSION: CLINICAL TRIALS ARE CRITICAL IN RENAL CARE
Clinical trials are critical in renal care. Not only can they improve health outcomes for patients, but the patients often are satisfied with their care and note a better quality of life.11 This process involves collaboration between patients, caregivers, and research institutions. A major barrier to clinical trials in kidney disease is that patients tend to not understand the benefits of participating. This issue can be overcome through open dialogue between patients and their clinical team.
Meet the Author
KURT MUSSINA, MBA
General Manager, Frenova Renal Research
Vice President of Clinical Studies Operations, Fresenius Medical Care North America
Chemist, executive and entrepreneur, Kurt Mussina leads FMCNA's contract research organization, a world-class network of more than 450 principal investigators across 260 research sites. A former analytical chemist and R&D scientist for Teva and Novartis pharmaceuticals, he holds his Bachelor of Science in chemistry from Montclair State University and his MBA from the Fuqua School of Business at Duke University.
References
- Bhatt A. Evolution of clinical research: a history before and beyond James Lind. Perspect Clin Res. 2010 January-March;1(1):6-10.
- Kaiser Associates. 2016 hot indications list. http://info.kaiserassociates.com/2016-hot- indications.
- Ibid.
- Shen J, Buechler A, Byrne J, Hecht J, James J, Pancratz B. Clinical research participation as a care option. PMG Research white paper, 2015. https://www.pmg-research.com/ Portals/0/Clinical%20Research%20as%20a%20Care%20Option_PMG%20Research%20 White%20Paper%20Web2.pdf.
- Violanti MT. So you want to work together: strengths and limitations of collaborative research. Commun Res Reports. 2009 June 6;16(4):377-385.
- Hague S, Snyder JR. Collaborative research: benefits and guidelines. J Allied Health. 1991;20(1): 69-73.
- Monteleone J. Patient recruitment: clinical research’s “white whale”? December 12, 2016. https://www.linkedin.com/pulse/patient-recruitment-clinical-researchs-white-whale-jason? articleId=7103206211285145337.
- Memorial Sloan Kettering Cancer Center. Despite pressing need, survey finds most Americans unlikely to enroll in clinical trials. Press release, May 23, 2016. https://www. mskcc.org/press-releases/despite-pressing-need-survey-finds-most-americans-unlikely- enroll-clinical-trials.
- Archived: Clinical research facts and figures for health professionals. Center for Information & Study on Clinical Research Participation. Accessed March 27, 2017. http://0393122. netsolhost.com/wp-content/uploads/2014/03/ciscrp_data_archive_facts_and_figures_for_ health_professionals.pdf.
- Stetka BS, Meunier CC. A novel approach to clinical trial recruitment. Medscape. July 29, 2014. http://www.medscape.com/viewarticle/828765.
- James J. Redesigning clinical trials: a new paradigm for drug development and care delivery. Open Forum. March 13, 2016. http://www.hbs.edu/openforum/openforum.hbs. org/goto/challenge/precision-medicine/redesigning-clinical-trials-a-new-paradigm-for- drug-development-and-care-delivery/comments-section.html.
- Majumdar SR, Roe MT, Peterson ED, Chen AY, Gibler WB, Armstrong PW. Better Outcomes for Patients Treated at Hospitals That Participate in Clinical Trials. Arch Intern Med. 2008;168(6):657-662. doi:10.1001/archinternmed.2007.124.



