THE REAL-WORLD EFFECTIVENESS OF VELPHORO IN PATIENTS ON CHRONIC DIALYSIS
Fresenius Kidney Care (FKC) has been studying the real-world effectiveness of Velphoro to see if a lower pill burden helps patients increase adherence and reduce their serum phosphorus levels. A retrospective analysis was conducted by the Medical Department of Fresenius Medical Care North America (FMCNA) Renal Therapies Group, in collaboration with practicing clinicians from various institutions. The study confirms that because Velphoro can reduce a patient’s pill burden by 50 percent or more, it can indeed be effective in helping patients reduce their serum phosphorus levels.
Hyperphosphatemia, elevated levels of serum phosphorus, is common among patients on dialysis.1,2 As kidney function declines, kidneys are increasingly unable to remove phosphorus, which leads to its accumulation in blood.3 High phosphorus levels cause bone disease and have been associated with increased cardiovascular morbidity and mortality.4,5,6 The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend a serum phosphorus (sP) target of 3.5-5.5 mg per dL for patients on dialysis.7 Oral phosphate binders (PBs), in combination with dialysis and dietary restriction of foods high in phosphate, are used to lower serum phosphorus levels; however, repeated reports over a decade indicate that 35 percent of patients on dialysis in the United States still have elevated serum phosphorus levels.8
Dialysis patients are prescribed many medications. The median daily pill burden assessed in 239 prevalent chronic dialysis patients was 19, and in 25 percent of subjects, it exceeded 25 pills per day.9 Phosphate binders accounted for about half of the daily pill burden, and 62 percent of patients were nonadherent to their binder medication.10 Higher PB pill burden associates with reduced adherence, which in turn is associated with poorer control of serum phosphorus.11,12,13
Velphoro (sucroferric oxyhydroxide), distributed by Fresenius Medical Care Renal Therapies Group, LLC, is a non-calcium, iron-based PB indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis. Phase III clinical trial data comparing Velphoro to a commonly used non-calcium PB, sevelamer, demonstrated that over a 52-week follow-up, control of serum phosphorus was maintained with significantly fewer Velphoro pills per day (3.3 pills per day) than sevelamer (8.7 pills per day).14
Since Velphoro was launched in the United States in 2014, database analyses have been conducted to determine the real-world effectiveness of Velphoro for several populations of FKC patients, including patients on hemodialysis (HD), peritoneal dialysis (PD), and home hemodialysis (HHD). De-identified clinical, demographic, and prescription data were extracted from FreseniusRx and FKC electronic records. These data analyses were retrospective and non-interventional, and had no influence on patient care or prescriptions. The analyses were conducted by the Medical Department of FMCNA Renal Therapies Group, in collaboration with practicing clinicians from various institutions. These analyses demonstrate that a higher percentage of patients receiving in-center HD, HHD, and PD who changed to Velphoro had improved sP control and reduced pill burden.
A short-term, six-month analysis of patients who switched to Velphoro showed that the proportion of patients achieving sP 5.5 mg per dL increased by 88 percent and 95 percent, respectively, during the first and second quarters after the switch. The mean prescribed PB pill burden was reduced from 9.6 to 4.0 pills per day (p < 0.001).15Velphoro led to improvements in patients achieving sP 5.5 mg per dL regardless of which previous PB was used (Figure 1).
A long-term analysis was performed on 4,925 dialysis patients with hyperphosphatemia (81.1 percent with sP > 5.5 mg per dL at baseline [BL]) switched from four different PBs to Velphoro for up to two years.16 Figure 2 shows the proportion of patients achieving sP 5.5 mg per dL on their prior PB (–1 period) and each quarter after switching to Velphoro. Among patients previously on sevelamer, there was a 135 percent increase in patients achieving sP 5.5 mg per dL with Velphoro (18 percent versus 42 percent) and a greater than 50 percent reduction in pill burden (10.5 to 4.4 pills per day, p < 0.0001). Similarly, when switched from calcium acetate, twice as many patients achieved sP 5.5 mg per dL and the pill burden reduced from 8.6 to 4.4 pills per day, p<0.0001. Interestingly, lanthanum carbonate users experienced up to a 71 percent increase in those achieving sP 5.5 mg per dL when switched to Velphoro, despite no reduction in pill burden (4.2 at BL to 4.5 Velphoro pills per day, p < 0.0001). Although shorter follow-up is available for ferric citrate, for patients who switched to Velphoro, there was a 36 to 50 percent increase in achieving sP 5.5 mg per dL and the pill burden was reduced from 6.2 pills per day to 4.4 pills per day (p < 0.0001).
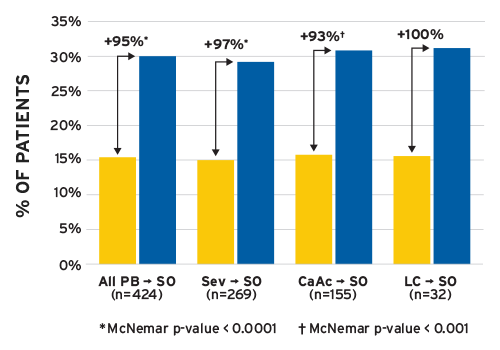
(SO = sucroferric oxyhydroxide (Velphoro), Sev = sevelamer, CaAc = calcium acetate, LC = lanthanum carbonate)
FIGURE 1 | Percentage of patients with sP 5.5 mg per dL at baseline and during months 4-6
Real-world data analyses for PD patients (n = 258) also showed improvements in sP levels along with reductions in PB pill burden after switching to Velphoro (Figure 3).17The proportion of patients achieving sP 5.5 mg per dL increased by 72 percent from baseline to month 6 of Velphoro follow-up. Prescribed PB pills per day decreased by 57 percent from 10 at BL to 4.3 at Velphoro follow-up (p < 0.0001).
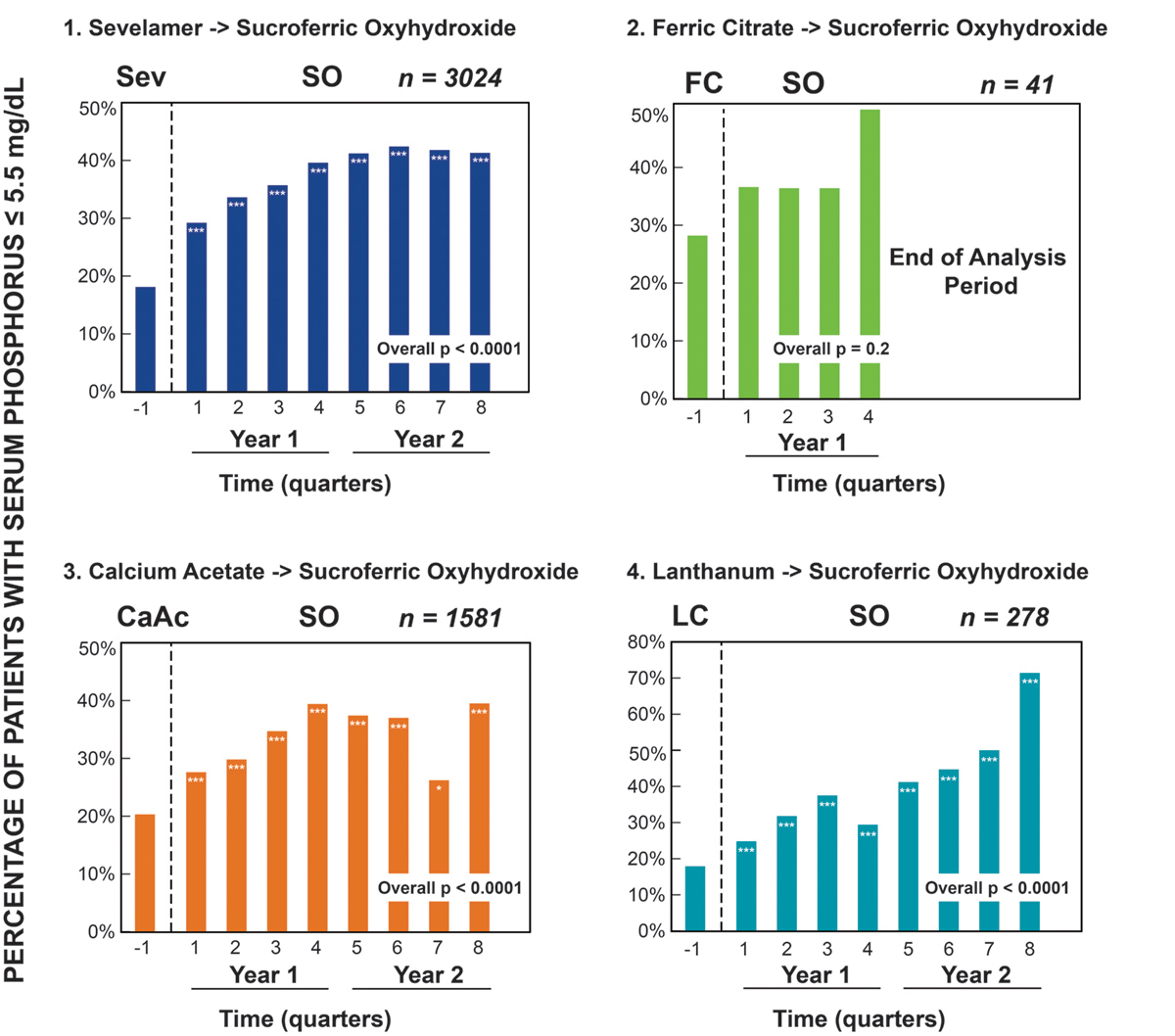
* p < 0.05; ** p < 0.001; *** p < 0.0001
FIGURE 2 | Two-year follow-up on Velphoro: percentage of patients with sP 5.5 mg per dL stratified by phosphate binder at baseline
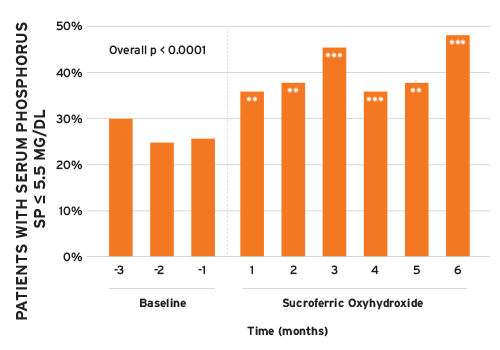
** p < 0.001; *** p < 0.0001
FIGURE 3 | Peritoneal dialysis patients with sP < 5.5 mg per dL by month (reference for statistical comparisons is month –1)
An analysis of 103 patients on HHD prescribed Velphoro for six months showed improvement in sP (Figure 4).18 Patients achieving sP 5.5 mg per dL increased by 96 percent, from 24.3 percent at baseline to 47.6 percent during Velphoro treatment. The number of PB pills per day was reduced from 8.9 to 4.3 pills per day.
Several sub-analyses were conducted to examine the effectiveness of Velphoro in specific HD patient populations. Among Hispanics/Latin Americans prescribed Velphoro, the percent of patients achieving sP < 5.5 mg per dL increased by 73 percent and the number of PB pills per day decreased by greater than 50 percent after six months.19 Black/African American patients prescribed Velphoro experienced a 58 percent increase in achieving sP < 5.5 mg per dL along with a greater than 50 percent reduction in number of PB pills per day.20 A subpopulation of patients with low serum albumin (< 3.5 g per dL) was analyzed to determine nutritional status and phosphorus control after switching to Velphoro.21 Serum albumin levels increased, while serum phosphorus levels decreased. These finding are being further investigated, as both low serum albumin and high serum phosphorus are known predictors of increased mortality risk.22,23 Sub-analyses of iron indices showed that changes were consistent with the findings reported in the phase III trial, where increases from baseline were not clinically significant and increases were mainly observed in patients receiving IV iron.24,25,26
In summary, significant increases (between 70 and 135 percent) in the number of patients achieving sP 5.5 mg per dL were observed for HD, HHD, and PD patients switched from any of the major binders (sevelamer, calcium acetate, lanthanum, and ferric citrate) to Velphoro, supporting the potency of Velphoro as a PB with a low pill burden. By reducing the PB pill number by half or more, Velphoro helps reduce the total daily pill burden and may improve adherence to medications. Ongoing and future data analyses include combination PB therapies, improved adherence, and assessing potential cost savings associated with lowering serum phosphorus (e.g., reducing hospitalization risk).27
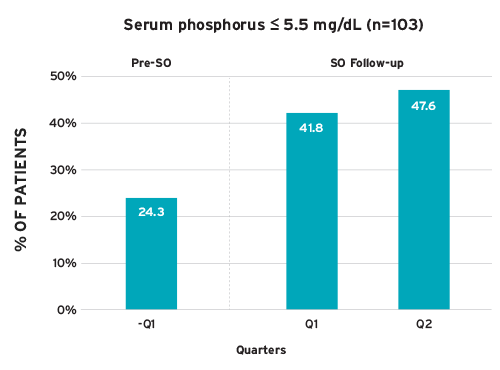
FIGURE 4 | Percent of home hemodialysis patients achieving serum phosphorus < 5.5 mg per dL after switched to Velphoro as part of routine clinical care (SO = sucroferric oxyhydroxide [Velphoro])
Meet Our Experts
Linda Ficociello, DSc
Director, Epidemiology, Fresenius Medical Care
In her seven years with Fresenius Medical Care Renal Therapies Group, Linda Ficociello has focused her research on evidence-based medicine and database studies on various topics, including bone and mineral metabolism and home dialysis therapies. She earned her bachelor’s degree at the University of New Hampshire, and her doctorate in epidemiology and master of science at Boston University School of Public Health. She completed her training as a post-doctoral fellow at Harvard Medical School.
Robert J. Kossmann, MD, FACP, FASN
Senior Vice President, Chief Medical Officer, Renal Therapies Group, Fresenius Medical Care
Robert “Rob” Kossmann practiced nephrology for two decades prior to joining FMCNA in 2014. The former president of the Renal Physicians Association and founding member of its Nephrology Coverage Advocacy Program, he served as nephrology advisor to the American Medical Association’s Relative Value Scale Update and founded the New Mexico Renal Disease Collaborative Group.
Claudy Mullon, PhD
Vice President, Medical Affairs & Clinical Research, Fresenius Medical Care
Claudy Mullon received his doctorate from Washington University in St. Louis and was a postdoctoral fellow at MIT where he remained a research affiliate for 10 years. He has co-authored more than 50 articles in peer-reviewed journals and is an inventor on 20 US patents. He is a current member of the American Society of Transplantation and an elected fellow of the American Institute for Medical and Biological Engineering.
References
The Real-World Effectiveness of Velphoro in Patients on Chronic Dialysis
by Linda Ficociello, DSc, Robert J. Kossmann, MD, FACP, FASN &
Claudy Mullon, PhD
- Joson CG, Henry SL, Kim S, et al. Patient-reported factors associated with poor phosphorus control in a maintenance hemodialysis population. J Ren Nutr 2016;26(3):141-48.
- Koiwa F, Yokoyama K, Fukagawa M, Terao A, Akizawa T. Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: a randomized, open-label, multicentre, 12-week phase III study. Nephrology (Carlton) 2017;22(4):293-300.
- Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med 2010;362:1312-24.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016;20(1):38-49.
- Locatelli F, Del Vecchio L, Violo L, Pontoriero G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf 2014;13(5):551-561.
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998;31(4):607-617.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 Suppl 3):S1-201.
- US DOPPS (Dialysis and Advanced CKD Outcomes and Practice Patterns Study Program) practice monitor: serum phosphorous (most recent). August 2017. http://www.dopps.org/dpm/.
- Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 2009;4(6):1089-96.
- Ibid.
- US DOPPS practice monitor. http://www.dopps.org/dpm/.
- Covic A, Rastogi A. Hyperphosphatemia in patients with ESRD: assessing the current evidence linking outcomes with treatment adherence. BMC Nephrol 2013;14:153.
- Fissell et al. Phosphate binder pill burden.
- Floege J, Covic AC, Ketteler M, et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 2015;30(6):1037-46.
- Coyne DW, Ficociello LH, Parameswaran V, Anderson L, Vemula S, Ofsthun NJ, Mullon C, Maddux FW, Kossmann RJ, Sprague SM. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: a retrospective analysis of pharmacy data. Clin Nephrol 2017;88(8):59-67.
- Coyne DW, Ficociello LH, Parameswaran V, Ofsthun NJ, Mullon C, Kossmann RJ, Sprague SM. Effectiveness of sucroferric oxyhydroxide in lowering serum phosphorus in 4,925 chronic hemodialysis patients. [Poster TH-PO1031] J Am Soc Nephrol 28: 2017.
- Kalantar-Zadeh K, Parameswaran V, Ficociello LH, Anderson L, Ofsthun NJ, Kwoh, C, Mullon C, Kossmann RJ, Coyne, DW. Real-world improvements in serum phosphorus levels and pill burden in peritoneal dialysis patients treated with sucroferric oxyhydroxide. Am J Nephrol 2018;47(3):153-161.
- Broumand V, Ficociello L, Li Y, Parameswaran V, Mullon C, Kossmann R. Serum phosphorus and phosphate binder pill per day in home hemodialysis patients prescribed sucroferric oxyhydroxide. Hemodialysis Abstracts from the 38th Annual Dialysis Conference March 3-6, 2018 Orlando, Florida. Hemodialysis International 2018;22(1):A1-A44.
- Henriquez M, Ficociello LH, Parameswaran V, Mullon C, Kossmann RJ. Phosphate binder pill burden and achievement of within-range serum phosphorus among Hispanic hemodialysis patients treated with sucroferric oxyhydroxide. Am J Kidney Dis 2017 April;69(4):A51. https://doi.org/10.1053/j.ajkd.2017.02.148.
- Ficociello LH, Ma L, Parameswaran V, Mullon C, Maddux FW, Kossmann RJ. Improved serum phosphorus control and decreased phosphate binder pill burden amongst African American hemodialysis patients taking sucroferric oxyhydroxide. FR-PO912. J Am Soc Nephrol 2015;26:576A.
- Kalantar-Zadeh K, Ficociello LH, Parameswaran V, Mondal H, Athienites NV, Mullon C, Kossmann RJ. Serum albumin and serum phosphorus among hemodialysis patients after initiating sucroferric oxyhydroxide. [Poster TH-PO1033] J Am Soc Nephrol 2017;28.
- Block et al. Association of serum phosphorus and calcium x phosphate product with mortality risk.
- Eriguchi R, Obi Y, Streja E, Tortorici AR, Rhee CM, Soohoo M, Kim T, Kovesdy CP, Kalantar-Zadeh K. Longitudinal associations among renal urea clearance-corrected normalized protein catabolic rate, serum albumin, and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2017;12(7):1109-17.
- Floege et al. Long-term effects of the iron-based phosphate binder.
- Velphoro® (sucroferric oxyhydroxide) prescribing information. Fresenius Medical Care North America. September 2014.
- Covic AC, Floege J, Ketteler M, Sprague SM, Lisk L, Rakov V, Rastogi A. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant 2017;32(8):1330-38.
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15(8):2208-18.


