IS OXYGEN SUPPLY TO VITAL ORGANS AT THE HEART OF KIDNEY PATIENTS’ DISEASE?
In a multiorgan organism, transport of gases (such as oxygen), nutrients, and metabolic waste products by diffusion-only processes is insufficient, so evolutionary forces honed elaborate delivery processes that adapted to our body’s needs. Oxygen is central to cellular respiration, the process that virtually all cells in our body use to make energy. Without oxygen delivery to cells, energy production would cease, the vital activities needed for life would end, and cells would die.
How Does The Body Deliver Oxygen from the Atmosphere to the Most Remote Territories in Our Bodies?
This feat of nature requires a complicated and highly regulated sequence of steps. An oxygen molecule needs to be taken up in the lungs by ventilation, which then diffuses across several membranes and other barriers in the lung alveolar space, into red blood cells (RBCs) that flow in the capillary bed surrounding these lung alveola. In the RBCs, four oxygen molecules bind to one of the 250 million hemoglobin molecules that reside in a single RBC, meaning that each healthy RBC carries around one billion oxygen molecules. The degree of oxygen binding to hemoglobin is called oxygen saturation and is expressed in percent of hemoglobin molecules carrying oxygen; in healthy subjects at sea level, the arterial oxygen saturation should be above 95 percent. The RBCs are then transported via the bloodstream to the most remote parts of our body. The level of this convective transport depends on the cardiac output, which is the blood volume ejected by our heart in a given time.
A few seconds after passage through the lungs, the RBCs arrive in the capillaries that serve tissues and organs, where oxygen is needed. Low tissue oxygen concentration, a consequence of oxygen consumption, in concert with other biochemical processes facilitate the unbinding of oxygen from hemoglobin. Now the “free” oxygen diffuses across multiple cell barriers and the interstitial space to its destination, which is the mitochondria inside the cells. Here, the oxygen molecules meet their fate and become part of water molecules generated by cellular respiration.
What Can Go Wrong with Oxygen Supply to Organs in Kidney Patients?
Diseases all along the oxygen supply route can affect oxygen delivery to tissues and organs (Figure 1). Disorders affecting the lungs and respiratory control can impact the uptake of oxygen from the atmosphere, and fluid overload with lung congestion and increased lung water may impede the diffusion of oxygen from the alveolar space to the RBCs.
Sleep disordered breathing (e.g., sleep apnea) is highly prevalent in patients with chronic kidney disease and on dialysis.1 Sleep disordered breathing not only results in reduced blood oxygen saturation (called hypoxemia, meaning oxygen saturation is below 90 percent), but also a host of other pathologies, such as high blood pressure and cardiac disease. Cardiac disease is frequent in chronic kidney disease and dialysis patients, and may result in poor cardiac output and consequently poor convective delivery of oxygen to the organs.
Uremic patients suffer from a peculiar reduction of density of capillaries in the heart (“capillary rarefication”).2 This condition increases the distance oxygen has to travel from the cardiac capillaries to the heart cells. The increased diffusion path further reduces oxygen delivery to these vital cells. In addition, fluid overload may increase the interstitial space and hence the distance between capillaries and cells, further reducing oxygen delivery. The latter pathology has been shown to affect the mucosal barriers that separate the bacteria-rich gut inner lumen from the blood side. There is evidence that a breakdown of this gut barrier may result in endotoxin transfer into the patient and consequent inflammation.
Figure 1 | Microcirculation in health and uremia.
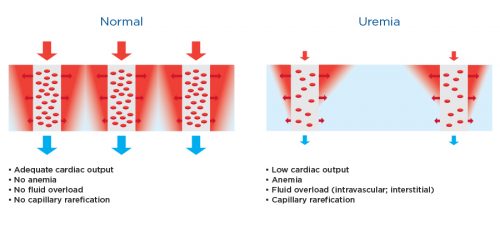
These schematic figures show the capillary bed (microcirculation) in healthy subjects (Left) compared to patients suffering from uremia (right). The red areas show those tissue regions with good oxygen supply (called a Krogh cylinder), while the blue show those regions with poor oxygen delivery. Note that regions with poor oxygen supply may e larger in kidney patients. Factors contributing to poor tissue oxygenation (hypoxia) are listed below the figures.
What Can We Do About Poor Oxygen Delivery to Organs?
The first step is making a proper diagnosis. It appears from the aforementioned biology that fluid overload and cardiac disease take center stage when it comes to poor oxygen delivery to tissues and organs. Clinical acumen and technology—in particular the Crit-Line monitor (CLM), imaging (echocardiography, X-ray), and bioimpedance—are used to arrive at a correct diagnosis.
While CLM measurements of hematocrit changes and relative blood volume are used on a daily basis with great success for fluid management, measurement of CLM oxygen saturation has only recently received wider attention. The CLM measures hematocrit and oxygen saturation 150 times per second in the extracorporeal circuit of the hemodialysis (HD) machine. In patients with an arteriovenous vascular access, the arterial oxygen saturation is measured, whereas in patients with dialysis catheters, the central venous oxygen saturation (ScvO2) is measured.
In a study of 982 HD patients at the Renal Research Institute (RRI), it was shown that patients who suffer from prolonged intradialytic hypoxemia (defined as an arterial oxygen saturation below 90 percent for more than one-third of the dialysis treatment time) have an increased risk of morbidity and mortality.3 Clinical observations in Fresenius Medical Care HD patients have also related intradialytic morbid events, such as hypotensive episodes, to low intradialytic oxygen saturation.4
Of note, ScvO2 correlates with perfusion of the upper body and can be used as a surrogate for cardiac output. At the 2016 Conference of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA), RRI researchers reported a study of 232 HD patients that demonstrated an association between low ScvO2 (and, by conjecture, low cardiac output) and mortality.5 Research is under way to explore the capability of CLM to diagnose intermittent hypoxemic episodes during dialysis, possibly related to sleep-induced breathing disorders. Ongoing CLM-related research explores the use of the device to quantitate upper body blood flow quasi-continuously during dialysis; first results of this research are encouraging (Figure 2).
Improving oxygen delivery to the tissues and organs depends on the underlying cause and requires a multifaceted approach (Figure 3). Fluid management and avoidance of intradialytic cardiac injury and/or myocardial stunning are important, as is anemia management to ascertain a sufficient blood oxygen carrying capacity. Treatment of hypoxemia during HD is the topic of an evolving study at RRI.
Figure 2 | Upper body blood blow (UBBF) during hemodialysis.
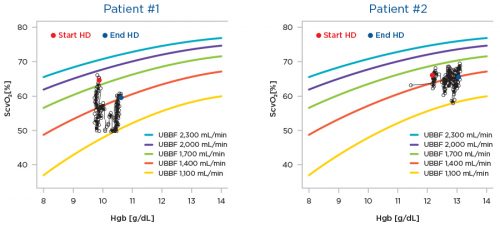
Conditions: arterial oxygen saturation 92%, O2 utilization 65 mL/min. This figure shows two HD Patients with central venous catheters as vascular access; the upper blood flow (UBBF) has been determined using CLM data. The x-axis shows the intradialytic hemoglobin levels (Hgb), thhe y-axis the ScvO2; both parameters are concurrently measured by the CLM. Patient #1 starts with a UBBF of around 2,000 mL/min and, after substantial swings, ends HD witth a UBBF of 1,400 mL/min. Patient #2 starts with UBBF of around 1,500 mL/min and end at 1,100 mL/min. This information provides minute-per-minute insights into the patient’s hemodynamic status.
Figure 3 | Means to improve oxygen supply to the tissues and organs.
Pathologies impacting oxygen supply to the tissues and organs, and potential therapeutic interventions (boxes).
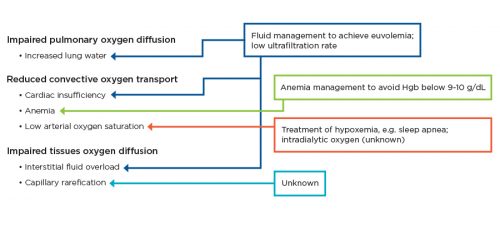
Pathologies impacting oxygen supply to the tissues and organs, and potential therapeutic interventions (boxes).
Meet the Expert
Peter Kotanko, MD, FASN
Research Director, Renal Research Institute
Fresenius Medical Care Renal Therapies Group
Noted researcher and scholar, Dr. Peter Kotanko heads research initiatives to improve patient outcomes and quality of life. An adjunct professor of medicine and nephrology at the Icahn School of Medicine at Mount Sinai in New York city, he has authored and co-authored more than 200 research papers and book chapters and is the former vice chair of Austria’s Hospital Barmherzige Brüder.
References
Is Oxygen Supply to Vital Organs at the Heart of Kidney Patients’ Disease? by Peter Kotanko, MD, FASN
- Unruh ML et al. Subjective and objective sleep quality in patients on conventional thrice- weekly hemodialysis: comparison with matched controls from the sleep heart health study. Am J Kidney Dis. 2008;52(2):305-13.
- Amann K et al. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9(6):1018-22.
- Meyring-Wosten A et al. Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol. 2016;11(4):616-25.
- Campos I et al. Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif. 2016;41(1-3):177-87.
- Chan et al. SP577, ERA-EDTA 2016 book of abstracts.



