REGENERATIVE MEDICINE: IMMEDIATE REALITY AND LONG-TERM PROMISE
The emerging field regenerative medicine holds both immediate and long-term promise for patients with ESRD. While many advances, including the development of bioengineered replacement kidneys, are still elusive goals, bioengineered vessels have more immediate potential to address the current shortcomings in vascular access. They are emerging as cost-effective, reliable, and safe alternatives to AVFs, catheters, and grafts.

Regenerative medicine is an emerging interdisciplinary field with dynamic potential clinical applications focused on the repair, replacement, or regeneration of dysfunctional cells, tissues, or organs from a range of problems, including congenital defects, disease, trauma, or aging. Regenerative medicine encompasses a combination of approaches, moving beyond traditional transplantation or replacement therapies. These methods include the use of soluble molecules, gene therapy, stem cell transplantation, tissue engineering, and reprogramming of cell and tissue types.1,2
Regenerative medicine holds the promise of regenerating damaged tissues and organs by stimulating previously irreparable organs to heal themselves. It also enables scientists to grow tissues and organs in the laboratory and safely implant them when a patient’s body cannot heal itself—allowing for “off the shelf” rationally designed tissues or organs. Taken to its limits, regenerative medicine has the potential to bridge the gap between the relatively small number of organs available for donation and the much larger number of patients in need of lifesaving organ transplantation.3
Regenerative medicine research has evolved into four broad areas of concentration:
- Medical devices and artificial organs: Despite the development of the artificial kidney decades ago, patients with end-stage renal disease (ESRD) need to be tethered to a machine several times each week unless they receive a kidney transplant. While the artificial kidney has been a lifesaver, the quality of life for the dialysis patient is certainly far from what can be considered normal. Regenerative medicine is working to improve quality of life. Already, new body parts have been created from a patient’s own cells and tissues. The success of laboratory-grown organs for transplant could eliminate the possibility of transplant rejection.
- Tissue engineering and biomaterials: Tissue engineers use many methods to promote the regrowth of cells lost to trauma or disease. These methods include the manipulation of artificial and natural materials that provide structure and biochemical instructions to young cells as they grow into specific kinds of tissue. These materials are called scaffolds because they provide support and materials for tissue regrowth.
- Cellular therapies: There are two ideas behind the use of cells as a medical treatment. The first is to provide a source of missing cells—for example, to heal a tissue that is injured or to renew a population of cells that are killed off by a disease such as Alzheimer’s. The second idea is to manipulate cells to produce a missing substance, such as a missing protein.
- Clinical translation: Clinical translation puts promising therapies into active trials. Every day, regenerative medicine is making great progress in the advancement of medicine. Once this new technology becomes widely used in clinical practice, the potential benefits to the US health care system and economy will be enormous. In the United States, national health expenditures grew 5.8 percent to $3.2 trillion in 2015, or $9,990 per person, and accounted for 17.8 percent of gross domestic product.4
Regenerative medicine has opened new avenues for curing patients with difficult-to-treat diseases and physically impaired tissues. Despite many successes, this field is still unfamiliar to many scientists and clinicians. This limitation is unfortunate, as tissue engineering and regenerative medicine could overcome the seemingly unsolvable problems faced by current medical treatments. Cell therapy and tissue engineering have the potential to revolutionize patient care. The key is transforming current scientific discoveries into novel and viable therapies.5
Patients with ESRD stand to benefit, potentially, from advances in regenerative medicine. Of course, the possibility of bioengineered replacement kidneys looms just beyond the horizon as a veritable disease-changing pot of gold. However, more tangible possibilities also exist, including the availability of bioengineered hemodialysis grafts that address many of the pitfalls in current hemodialysis access care (Figure 1).

Patients undergoing hemodialysis require reliably patent, infection-free vascular accesses in order to receive life-sustaining dialysis. During the last 50 years that hemodialysis has been in use, the arteriovenous fistula (AVF) has emerged as the access option of choice for these patients. Yet, as the most recent available data reveals, unacceptably high numbers of these accesses never mature to the point of being useful.6 Furthermore, even AVFs that finally mature to the point of being useful require a median of 111 days before they can be used. This exposes patients to the unacceptably high morbidity and mortality rates of catheter-based dialysis, and likely contributes to the high annual cost of ESRD care ($34 billion as of 2015).7,8,9 While expanded polytetrafluoroethylene (ePTFE) grafts provide some benefit in that they do not require long periods of time to mature, their relatively high thrombosis and infection rates preclude these as optimal solutions to the access conundrum faced by this population.10
Bioengineered vessels have emerged as cost-effective, reliable, and safe alternatives to AVFs , catheters, and grafts. Specifically, bioengineered human acellular vessels—such as those produced by Humacyte, Inc., for example—appear to address many of the current shortcomings in vascular access. Data from a recent phase II trial reviewed the placement of 60 such grafts (in two different study arms) and revealed no infections of the accesses themselves; less time-to-use than the current median time-to-use for AVFs ; and increased primary, primary-assisted, and secondary patencies at six months when compared with ePTFE grafts.11 Furthermore, since the bioengineered grafts consist of acellular matrixes, no instance of host immune rejection was reported, and one study participant even underwent successful renal transplantation during the study.12 As of Q1 2018, these grafts have responded well to the rigors of two thrice-weekly needle punctures.13,14
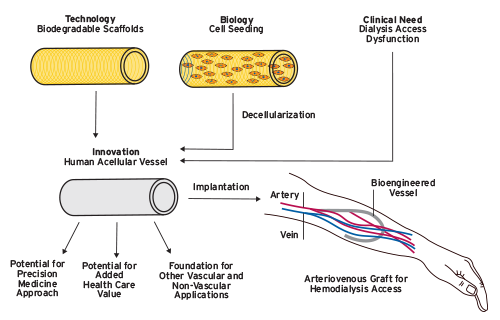
Figure 1 | Bioengineered human acellular vessels: an innovation in haemodialysis. Development of the human acellular vessel is an example of combining technological and biological advances to address an unmet clinical need. If shown to be efficacious, this new technique could have important ancillary benefits such as enabling precision medicine approached and increasing health-care value for patients on haemodialysis as well as potential uses in other vascular and non-vascular applications.
Source: P. Roy-Chaudhury, Dialysis: bioengineered vessels for dialysis access: soon to be a reality?Nat Rev Nephrol 2016;12(9):516-17.
The imaging appearance of humacyte grafts in the limited available experience to date provides no characteristic features to suggest the access is a humacyte graft. For example, while on ultrasound, humacyte grafts can be distinguished from ePTFE grafts by the absence of hyperechoic (bright white) lines outlining the graft (Figures 2 and 3), these features are also seen in fistulas. And, the fluoroscopic appearance of humacyte grafts is identical to that of ePTFE grafts (Figure 4). Therefore, clinical history is critical since angioplasty balloon sizes greater than 6mm and mechanical thrombectomy devices (all common tools for the interventionalist) are contraindicated in humacyte grafts that require intervention.
If further trials continue to show the benefits reported in this phase II study, bioengineered vessels such as these could represent a needed value proposition in the costly ESRD world and would begin to fulfill the promise of regenerative medicine. One can imagine a day in the not-too-distant future when “off the shelf” bioengineered acellular coronary artery and other bypass grafts will be used to treat other pervasive vascular diseases.
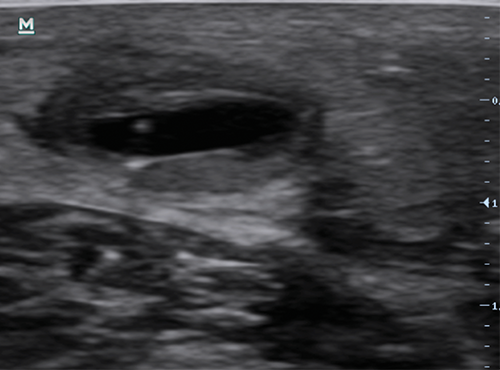
Figure 2 | Angled longitudinal ultrasound image of a needle being used to access a conventional ePTFE graft. Note the hyperechoic (bright) borders outlining the graft. The bright dot towards the top is the tip of the needle being used to access the graft.
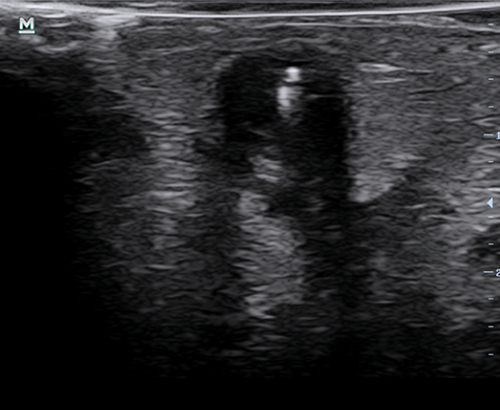
Figure 3 | Transverse ultrasound image of a needle being used to access a humacyte graft. Note the lack of a defined border visible by ultrasound; this appearance is similar to that of a fistula. The bright line coming from superior aspect is the needle.
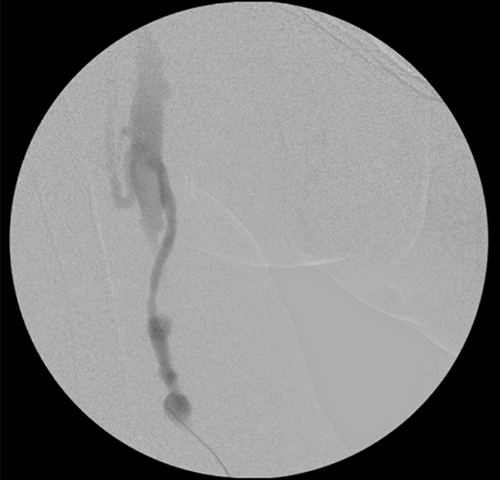
Figure 4 | Fluoroscopic image from a fistulagram showing a humacyte graft with puncture-site pseudoaneurysms. The access point is just below the lowest pseudoaneurysm. The fluoroscopic appearance of a humacyte graft is indistinguishable from that of a conventional graft.
Meet Our Experts
Murat Sor, MD
Chief Medical Officer, Azura Vascular Care
A graduate of the University of Pennsylvania, Murat Sor is certified by the American Board of Radiology, with subspecialty certification in interventional radiology. He is an assistant professor at Georgetown University Hospital’s Interventional Radiology Fellowship program and an adjunct instructor at the George Washington University School of Medicine.
Warren S. Krackov, MD, MA, MS
Medical Director, Vascular Interventions of Tampa
Warren S. Krackov is an interventional radiologist who specializes in dialysis access management and was a pioneer in performing transradial uterine fibroid embolization in the outpatient setting. He performs a full range of dialysis access procedures, including treatments for critical maintenance and management for a dialysis patient’s accesses. He serves on the Medical Advisory Board of Azura Vascular Care.
Catherina Madormo, RN, BSN
Clinical Research Manager, Azura Vascular Care
Catherina (Kitty) Madormo supports the work of the chief medical officer, the Medical Advisory Board, and Azura physicians involved with the network’s clinical research activities. She serves as the point of contact between Azura and other FMCNA research entities and works with Azura’s marketing team as a clinical subject matter resource. She earned her bachelor’s degree in nursing from Seton Hall University and has been involved in the care and treatment of nephrology patients throughout her career.
References
Regenerative Medicine: Immediate Reality and Long-Term Promise
by Murat Sor, MD, Warren S. Krackov, MD, MA, MS & Catherina Madormo, RN, BSN
- Greenwood H, Thorsteinsdottir H, Perry G, et al. Regenerative medicine: new opportunities for developing countries. Int J Biotechnol 2006;8(1-2):60-77.
- Mason C, Dunnill P. A brief description of regenerative medicine. Regen Med 2008;3(1):1-5.
- National Institutes of Health. Regenerative Medicine Fact Sheet. Accessed March 1, 2018. https://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=62.
- RegenerativeMedicine.net. What is regenerative medicine? Accessed March 1, 2018. http://www.regenerativemedicine.net/What.html.
- Sampogna G, Yousuf S, Forgione A. Regenerative medicine: historical roots and potential strategies in modern medicine. J Microsc Ultrastruct 2015;3(3):101-7. doi :10.1016/j.jmau.2015.05.002.
- United States Renal Data System. “Vascular Access,” chap 3 in 2017 annual data report. https://www.usrds.org/2017/view/v2_03.aspx.
- Ibid.
- USRDS. 2017 annual data report highlights. https://www.usrds.org/adrhighlights.aspx.
- Roy-Chaudhury P. Dialysis: bioengineered vessels for dialysis access: soon to be a reality? Nat Rev Nephrol 2016;12(9):516-17. doi :10.1038/nrneph.2016.119.
- Ibid.
- Lawson JH, Glickman MH, Ilzecki M, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 2016;387(10032):2026-34. doi :10.1016/S0140-6736(16)00557-2.
- Ibid.
- Ibid.
- Tillman BW, Yazdani SK, Neff LP, et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg 2012;56(3):783-93. doi :10.1016/j.jvs.2012.02.030.


