EXPLORING THE ROOT CAUSE OF SECONDARY HYPERPARATHYROIDISM: A VIRTUAL CINACALCET TRIAL
Due to high pill burden and gastrointestinal side effects, patient adherence to daily intake of cinacalcet is limited. To explore alternate ways of increasing adherence, Fresenius Medical Care North America (FMCNA), Renal Research Institute (RRI), and Fresenius Medical Care (FMC) Global Research and Development (GRD) created an innovative virtual trial that not only approximated drug behavior and disease progression, but also reflected human behavior.
Decline in kidney function affects every single cell in the body, from heart to skin, from brain to gut, from liver to muscles. Bones are among the first organ systems to be impacted by the reduced excretion of solutes, such as phosphorus. Also, the production of the hormone calcitriol by the kidneys is reduced, resulting in less intestinal calcium absorption. In concert, the rise in phosphate, the lower calcitriol levels, and the lower calcium absorption from food stimulate the parathyroid gland to produce more parathyroid hormone (PTH).
PTH’s main function is to maintain stable blood calcium levels within a narrow range; within seconds of a decline in serum calcium, the secretion of PTH increases sharply. PTH promotes the mobilization of calcium from the bone, the synthesis of calcitriol, and the excretion of phosphate by the kidneys (Figure 1). When the kidneys fail, these functions fail, and a vicious cycle ensues, resulting in sustained high PTH levels, a condition called secondary hyperparathyroidism (SHPT). In the long term, these elevated PTH levels have deleterious effects on bone health and, indirectly through not yet fully understood mechanisms, on blood vessels.
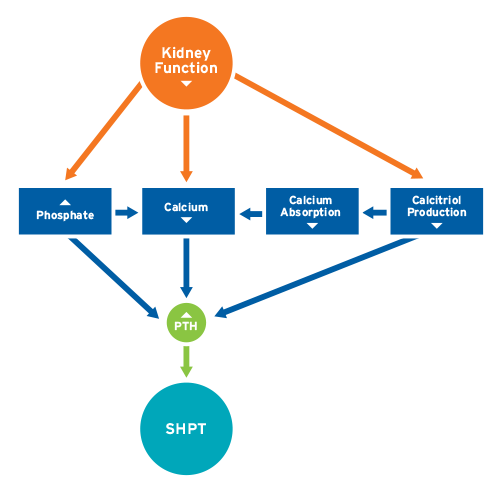
Figure 1 | Pathophysiology of secondary hyperparathyroidism (SHPT)
Current Therapies for SHPT: Phosphate Binders, Vitamin D Analogues, and Calcimimetic Drugs
Having this pathophysiology of SHPT in mind, one can easily understand that the therapeutic approach is multipronged and entails several strategies. They include dietary phosphate restriction and the use of phosphate binders to reduce the phosphate absorption from the gut; using calcitriol and vitamin D analogues (e.g., paricalcitol) to lower the PTH secretion and increase gut calcium absorption; and using calcimimetic drugs that sensitize the parathyroid gland to the effects of calcium and thus reduce the secretion of PTH. Cinacalcet (Sensipar®) was approved by the Food and Drug Administration in 2004 for the treatment of SHPT. Per its label, it should be taken daily with food or shortly after a meal. Unfortunately, due to high pill burden and gastrointestinal side effects, patient adherence is limited, with drug refill rates between 37 and 53 percent.1
Against that background, we asked ourselves how a thrice-weekly guaranteed intake of cinacalcet, combined with a snack at the end of the hemodialysis session, would compare with the empirically observed poor adherence administration scheme. More specifically, do population PTH levels differ between these two scenarios? Since a literature review failed to provide satisfactory evidence to answer that question, scientists embarked on a virtual clinical trial.
What is a Virtual Clinical Trial?

Virtual clinical trial simulation is a frequently used tool in drug development to, among other things, determine appropriate dosing regimens. Realistic simulations provide valuable information regarding safety and limitations of suggested treatment protocols by generating clinical responses of virtual subjects. Fresenius Medical Care’s Global Research & Development and RRI have developed cutting-edge methodologies to create highly reliable and realistic virtual clinical trials.2 Virtual subjects are designed to not only approximate the disease progression and drug kinetics and dynamics in patients, but also reflect human behavior, such as adherence to drug administration.
Brief Overview of the Physiologically Based PTH and Cinacalcet Model
To be able to realistically simulate disease progression and drug behavior in patients with SHPT, RRI designed a physiologically based mathematical model (Figure 2).3 The model describes in detail the life cycle of PTH-producing parathyroid gland cells and the secretion of PTH. Furthermore, mechanisms associated with conditions like hypocalcemia and hyperphosphatemia (both common in kidney patients) that affect calcium-sensing receptors and vitamin D receptors are accounted for in the model.
A pharmacokinetic model specific to cinacalcet was developed by RRI that includes, among other things, a “first pass effect.” This is a phenomenon of drug metabolism related to the liver and gut wall where a large fraction of a drug is lost during the process of absorption. Additionally, an operational model of allosterism is used to simulate the pharmacodynamic effect of cinacalcet on the calcium-sensing receptor.4
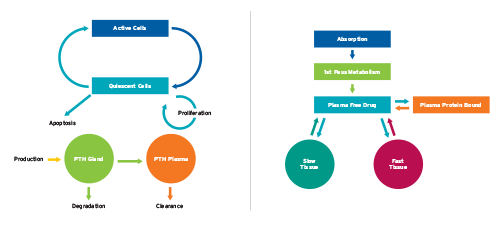
Figure 2 | Sketches of the physiologically based PTH model and the cinacalcet pharmacokinetic and pharmacodynamic model developed by RRI
Results of the Virtual Clinical Trial
The virtual clinical trial demonstrated that a reliable thrice-weekly administration of cinacalcet, e.g., after the end of a hemodialysis session (Figure 3), has a materially identical effect on lowering PTH levels compared with a prescribed daily cinacalcet administration with limited patient adherence.
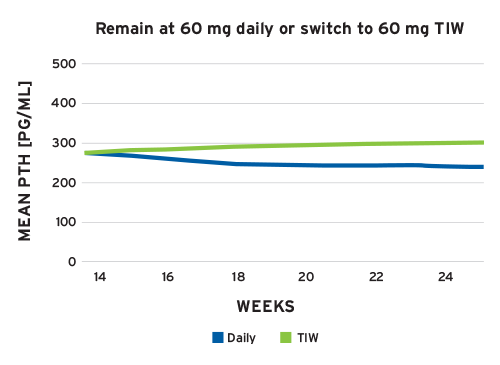
Figure 3 | Simulation of daily 60 mg and thrice-weekly (TIW) 60 mg cinacalcet administration for patients after a 12-week cinacalcet run-in phase with daily administration
Outlook

The virtual clinical trial provides a powerful and readily available tool to analyze the impact of various dosing regimens and adherence factors on the PTH-lowering effects of cinacalcet administration. It is also an effectual tool to support decision making regarding the future use of calcimimetic drugs by Fresenius Kidney Care patients.
Meet Our Experts
Doris H. Fuertinger, MSC, PHD
Director, Biomedical Modelling and Simulation, Global Research and Development, Fresenius Medical Care
Doris Fuertinger received her master's degree in mathematics in 2008 and a doctorate in applied mathematics in 2012. She pioneered the concept of a virtual dialysis clinic and virtual clinical trials, and together with her research team developed methods to apply them to the area of bone mineral metabolism, anemia, and fluid management of dialysis patients. She has authored and co-authored multiple papers and book chapters, and is inventor on a number of international and US patents held by FMC.
Peter Kotanko, MD, FASN
Research Director, Renal Research Institute
Noted researcher and scholar, Peter Kotanko heads research initiatives to improve patient outcomes and quality of life. An adjunct professor of medicine and nephrology at the Icahn School of Medicine at Mount Sinai in New York City, he has authored and co-authored more than 250 research papers and book chapters, and is the former vice chair of a medical department at an academic teaching hospital in Graz, Austria.
References
Exploring the Root Cause of Secondary Hyperparathyroidism: A Virtual Cinacalcet Trial
by Doris H. Fuertinger, MSc, PhD & Peter Kotanko, MD, FASN
- Gincherman Y, Moloney K, McKee C, Coyne DW. Assessment of adherence to cinacalcet by prescription refill rates in hemodialysis patients. Hemodial Int 2010 Jan;14(1):68-72. doi:10.1111/j.1542-4758.2009.00397.x.
- Fuertinger DH, Topping A, Kappel F, Thijssen S, Kotanko P. The virtual anemia trial: an assessment of model-based in silico clinical trials of anemia treatment algorithms in patients with hemodialysis. CPT Pharmacometrics Syst Pharmacol 2018 Jan 25. doi:10.1002/psp4.12276.
- Schappacher-Tilp G, Preciado P, Maheshwari V, Cherif A, Fuertinger DH, Thijssen S, Bushinsky D, Kotanko P. A mathematical model of parathyroid gland biology in uremic patients. J Am Soc Nephrol 2017 (abstract supplement), 28:A473.
- Leach K, Gregory KJ, Kufareva I, et al. Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor. Cell Research 2016;26(5):574-92. doi:10.1038/cr.2016.36.



