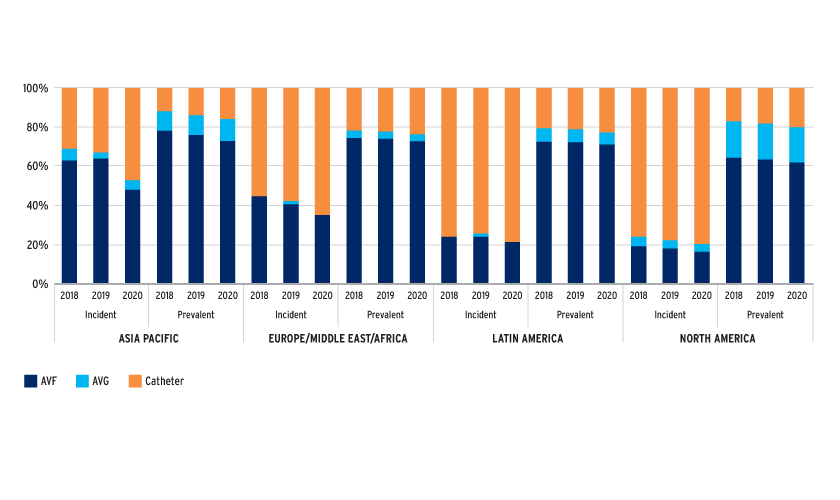
FIGURE 1 | Catheter use in incident and prevalent patients
HIGH CATHETER USE IN HD PATIENTS
Catheter use is high, especially in incident patients, despite the knowledge that arteriovenous vascular access is superior and despite initiatives focusing on increasing AVF use. USRDS and DOPPS data suggest that better transition (pre-HD) care planning that includes vascular access care improves arteriovenous access rates.3,4 It is noteworthy that over 90 percent of AVFs are cannulated within one month in Japan, whereas only 70 percent of AVFs are cannulated within four months in the U.S.5 This observation may reflect the differences in success rates of AVF creation surgeries between these countries. Regardless of the high catheter rates, AVG usage remains low in incident patients, especially in the EMEA region.
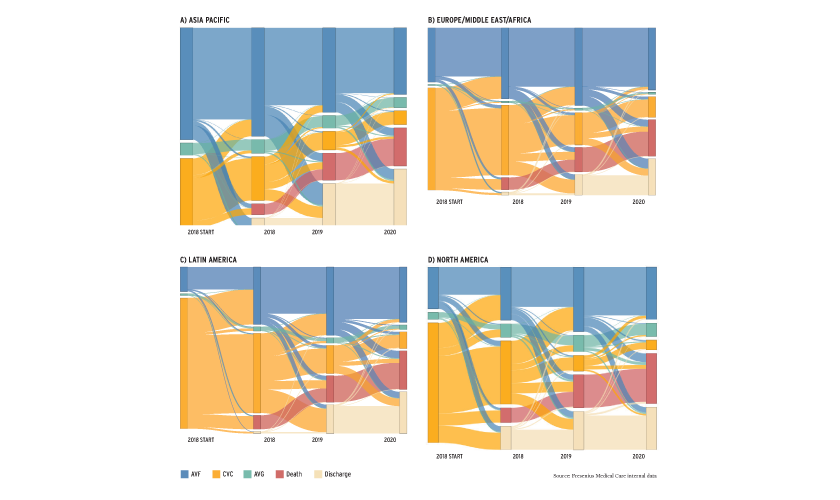
Regional variation is due to numerous factors including economic differences, patient characteristics, healthcare systems, and physician practice patterns. The Sankey diagrams (Figure 2) depict the outcomes of individuals who started HD in 2018 and serve as a stark reminder of the risks of initiating HD with a catheter.6 Selection bias alone may not explain these findings.7,8,9 The pandemic’s impact on vascular access outcomes may have long-term effects on patient outcomes and healthcare costs.
FIGURE 2 | Sankey diagrams (A-D) demonstrating evolution of outcomes for those initiating HD in 2018 by vascular access type. Individual colors represent unique outcomes, and the width of the bands represents proportion of individuals with that access and/or outcome.
EVOLVING APPROACHES TO VASCULAR ACCESS CARE
The high utilization of HD catheters, notwithstanding significant policy and practice changes, has prompted increased focus on catheter avoidance strategies, such as the catheter-last approach, which incorporates patient-centered and personalized care.10,11,12,13
Application of patient-centered care to minimize catheter burden requires, first, enhanced patient risk prediction algorithms that can more accurately predict end stage kidney disease risk, timing, and arteriovenous vascular access success. Using these predictions and considering the healthcare systems, care approaches that allow treatment personalization by judicious and appropriate use of the available therapeutic armamentarium are needed, and those that are most suited to the patient’s vascular access care needs considering their physical and biological characteristics. This should include consideration of AVGs, early cannulation graft materials, human-derived vascular conduits, percutaneous AVFs, and peritoneal dialysis (PD). As an example, an elderly individual who needs HD sooner and has high predicted AVF maturation failure risk may benefit from the use of AVGs, humanized vascular conduits, or indeed PD, depending on the goals and preferences of the individual.
Furthermore, it is important to prolong patency of arteriovenous access by more accurately predicting vascular access failure and having timely and effective interventions to maintain patency. In some circumstances, the approach may include creation of a new dialysis access, ideally prior to complete failure of the vascular access in use.
Timely referral for arteriovenous access creation is one of the critical steps needed to avoid the use of catheters for HD initiation. To this end, FME-EMEA has developed the Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD). PROGRES-CKD integrates 32 routinely collected clinical parameters into one summary risk score to predict kidney replacement initiation within six months with excellent accuracy (AUC=90 percent). PROGRES-CKD was deployed in the Czech Republic and Italy as an integral part of RenalSafeguard®, a holistic care program aimed at preventing CKD progression, reducing cardiovascular risk, and improving transition management for NDD-CKD patients.
One additional pillar of decision making is accurate prediction of arteriovenous access success, pre- and post-creation, based on predictive algorithms that are able to incorporate broad data sources such radiological, physiological, sensor-based, metabolomics, and genomics data. FME-AP and the Renal Research Institute (RRI) collaborated with the UK-based Manchester Vascular Access (MANVAS) study to leverage the combination of metabolomics data with clinical, ultrasound, and laboratory information to predict AVF success, prior to creation.14 Post-creation AVF maturation can be monitored with a non-invasive and semi-continuous monitoring approach developed by RRI that measures central venous oxygen saturation (ScvO2) and estimates upper body blood flow (eUBBF) using the Crit-Line® Monitor. The study demonstrated a clear relationship between AVF maturation and ScvO2 as well as eUBBF temporal dynamics, thus allowing for personalized cannulation and intervention plans.15
EVOLUTION IN ARTERIOVENOUS ACCESS CREATION
Innovative technologies introduced in recent years offer new options for vascular access care.16 Foremost, several versions of early cannulation grafts are readily available and allow for cannulation within 72 hours of implantation. Early cannulation grafts may have primary and secondary patency and rates of infection, pseudoaneurysms, and thrombotic events that are comparable to polytetrafluoroethylene grafts.17
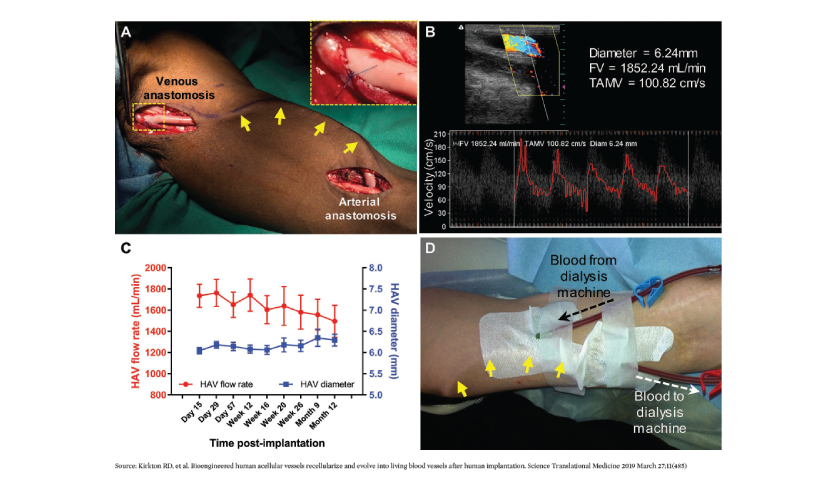
The latest advancement in the vascular access arena is the human acellular vessel (HAV) by Humacyte Inc.18,19 The HAV is a bioengineered vessel cultivated and decellularized from antigens prior to implantation. The HAV can be readily shipped, refrigerated, and inserted when needed. After implantation, the HAV has been shown to adopt characteristics of native blood vessels (Figure 3). This is a first-of-its-kind technology and promises to provide patients with a truly personalized vascular access option lined by their own vascular cells, and is available off the shelf. In the right circumstances, the HAV may be considered a first-line vascular access option, one with no immune-reactivity and with low infection rates. The HAV is also being studied for other indications including vascular trauma, peripheral arterial disease, and pediatric cardiac surgery.
FIGURE 3 | Clinical implantation and hemodialysis access. (A) Intraoperative image of HAV implantation (tunneled under the skin; yellow arrows) in the upper arm for use as a dialysis access. (B and C) Postoperative Doppler ultrasound measurements of mid-HAV diameter, blood flow volume (FV), and time averaged mean velocity (TAMV) during follow-up within the patient population. (D) Photograph of cannulation of the subdermal HAV (yellow arrows) for hemodialysis.
Lastly, the advent of percutaneous AVF creation has greatly enhanced and expedited AVF creation options for individuals on dialysis. Two such approaches are currently offered, and with the appropriate anatomy, a fistula can be created in an outpatient setting without the need for admission to the hospital. Current studies from percutaneous devices (Wavelinq by Bard and Ellipsys by Avenu) indicate high technical success rates, with primary patency rates equivalent to surgically created AVFs.20,21,22 The advantages of a percutaneous AVF include the absence of surgical scars, early creation, and shorter time to surgery. However, AVFs created using percutaneous techniques may require specialized cannulation approaches.
EVOLUTION IN ARTERIOVENOUS ACCESS MAINTENANCE
Annually, 14 percent of AVFs and between 50 and 80 percent of AVGs fail acutely, primarily as a result of thrombosis associated with stenotic lesions.23 Prevention of vascular access thrombosis is beneficial for many reasons, and thrombosis itself, regardless of other lesions, portends poor vascular access survival.24
Vascular access surveillance techniques such as access flow (Qa) measurements are commonly performed using dilution techniques. Despite the wide use of Qa measurements, these measurements have key limitations, including the need for special equipment, insensitivity to hemodynamic changes during dialysis, and inaccurate detection of outflow stenosis.25
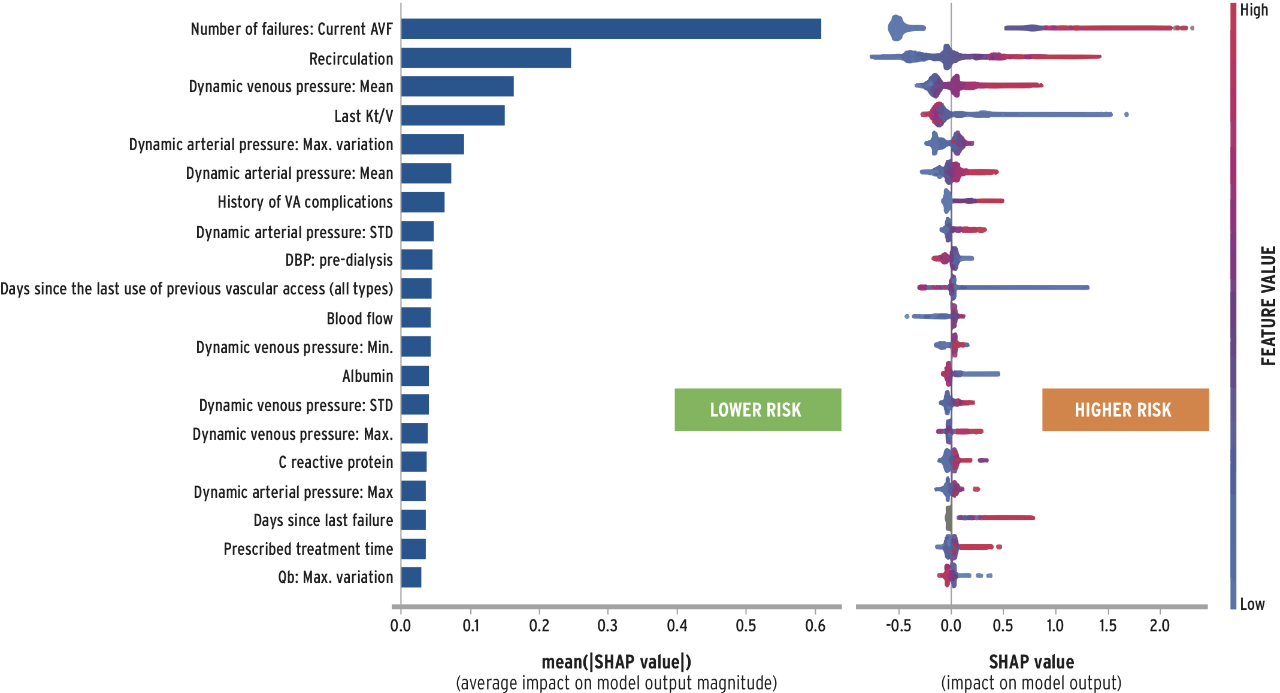
To enable personalized AVF management, Fresenius Medical Care is currently developing several applications of artificial intelligence to enhance surveillance and assist in medical decision making. FME-EMEA has trained and validated a risk stratification algorithm to predict AVF dysfunction. The AVF failure score (AVF-FS) has excellent discrimination properties (AUC=0.81) and is sensitive to changes in AVF functional parameters. The AVF-FS can be used to predict AVF failure based on routinely collected information and machine sensor data. In addition, the model may offer diagnostic clues based on ranking of variable risk impact (Figure 4). Additional advancements include stenosis detection by e-stethoscope and other sensor records and image recognition algorithms detecting and grading AVF aneurysms.26
FIGURE 4 | An example of AVF-FS model output depicting the ranking of variable importance and its association with the failure risk
Once detected, stenoses are primarily managed using endovascular techniques. Drug-coated balloons have shown promise in reducing re-stenosis rates in vascular access.27,28 Newer drug coatings are also being evaluated.29
The calls for patient-centered and personalized vascular access care are welcome. The evolving approaches to vascular access decision making, creation, and preservation will contribute to a brighter future for individuals living with kidney disease.
Meet The Experts
References
- Tong A, Manns B, Hemmelgarn B, et al. Standardised outcomes in nephrology–haemodialysis (SONG-HD): study protocol for establishing a core outcome set in haemodialysis. Trials 2015;16:364.doi:10.1186/s13063-015-0895-7.
- United States Renal Data System. USRDS 2020 annual data report: epidemiology of kidney disease in the United States. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
- Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol 1996;7(4):523-35. doi:10.1681/ASN.V74523.
- USRDS 2020 annual data report: epidemiology of kidney disease in the United States.
- Pisoni RL, Zepel L, Port FK, et al. Trends in US vascular access use, patient preferences, and related practices: an update from the US DOPPS Practice Monitor with international comparisons. Am J Kidney Dis 2015;65(6):905-15. doi: 10.1053/j. ajkd.2014.12.014.
- Ibid.
- USRDS 2020 annual data report: epidemiology of kidney disease in the United States.
- Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol 2004 Feb;15(2):477-86. doi: 10.1097/01.asn.0000109668.05157.05.
- Arhuidese IJ, Orandi BJ, Nejim B, Malas M. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg 2018;68(4):1166-74. doi:10.1016/j.jvs.2018.01.049.
- Grubbs V, Wasse H, Vittinghoff E, et al. Health status as a potential mediator of the association between hemodialysis vascular access and mortality. Nephrol Dial Transplant 2014;29(4):892-98. doi: 10.1093/ndt/gft438.
- Gomes A, Schmidt R, Wish J. Re-envisioning Fistula First in a patient-centered culture. Clin J Am Soc Nephrol 2013;8(10):1791-97. doi:10.2215/CJN.03140313.
- Lacson E, Lazarus JM, Himmelfarb J, et al. Balancing fistula first with catheters last. Am J Kidney Dis 2007;50(3):379-95. doi:10.1053/j.ajkd.2007.06.006.
- Lok CE, Huber TS, Lee T, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. Am J Kidney Dis 2020;75(4 Suppl 2):S1-S164. doi:10.1053/j. ajkd.2019.12.001.
- Wish JB. Catheter last, fistula not-so-first. J Am Soc Nephrol 2015;26(1):5-7. doi:10.1681/ASN.2014060594.
- Nikam M. Clinical investigation of the arteriovenous access for haemodialysis (doctoral thesis, The University of Manchester, United Kingdom, 2014), https://www.research.manchester.ac.uk/portal/files/54552626/FULL_TEXT.PDF.
- Rosales LM, Zhang H, Mateo M, et al. Tracking arteriovenous fistula maturation: a novel approach. Blood Purif 2019;47(1-3):240-45. doi:10.1159/000494742.
- Agarwal AK, Haddad NJ, Vachharajani TJ, Asif A. Innovations in vascular access for hemodialysis. Kidney Int 2019;95(5):1053-63. doi:10.1016/j.kint.2018.11.046.
- Shakarchi JA, Inston N. Early cannulation grafts for haemodialysis: an updated systematic review. J Vasc Access 2019;20(2):123-27. doi:10.1177/1129729818776571.
- Niklason LE, Lawson JH. Bioengineered human blood vessels. Science 2020;370(6513):eaaw8682. doi:10.1126/science.aaw8682.
- Wang J, Lawson JH, Niklason LE, et al. “Bioengineered human acellular vessels,” in Tissue-Engineered Vascular Grafts, ed. BH Walpoth, et al. (Springer International Publishing, 2020), 549-74.
- Beathard GA, Litchfield T, Jennings WC. Two-year cumulative patency of endovascular arteriovenous fistula. J Vasc Access 2020;21(3):350-56. doi:10.1177/1129729819877780.
- Inston N, Khawaja A, Tullett K, Jones R. WavelinQ created arteriovenous fistulas versus surgical radiocephalic arteriovenous fistulas? A single-centre observational study. J Vasc Access 2020;21(5):646-51. doi:10.1177/1129729819897168.
- Mallios A, Bourquelot P, Franco G, et al. Midterm results of percutaneous arteriovenous fistula creation with the Ellipsys Vascular Access System, technical recommendations, and an algorithm for maintenance. J Vasc Surg 2020;72(6):2097-2106. doi:10.1016/j.jvs.2020.02.048.
- Huijbregts HJ, Bots ML, Wittens C, et al. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 2008;3(3):714-19. doi:10.2215/CJN.02950707.
- Nikam MD, Ritchie J, Jayanti A, et al. Acute arteriovenous access failure: long-term outcomes of endovascular salvage and assessment of co-variates affecting patency. Nephron 2015;129(4):241-46. doi: 10.1159/000375500.
- Paulson WD, Moist L, Lok CE. Vascular access surveillance: an ongoing controversy. Kidney Int 2012;81(2):132-42. doi:10.1038/ki.2011.337.
- Krackov W, Sor M, Razdan R, et al. Artificial intelligence methods for rapid vascular access aneurysm classification in remote or in-person settings. Blood Purif 2021;1-6. doi:10.1159/000515642.
- Irani FG, Tan BS. Drug coated balloons: are they the holy grail for dysfunctional dialysis arteriovenous fistulas? Cardiovasc Intervent Radiol 2021;44(3):516-17. doi:10.1007/s00270-020-02690-4.
- Lookstein RA, Haruguchi H, Ouriel K, et al. Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 2020;383(8):733-42. doi:10.1056/NEJMoa1914617.
- Tan CW, Tan RY, Pang SC, et al. Single-center prospective pilot study of sirolimus drug-coated balloon angioplasty in maintaining the patency of thrombosed arteriovenous graft. J Vasc Interv Radiol 2021;32(3):369-75. doi:10.1016/j.jvir.2020.11.010.